Improving Point-of-Need Testing with Lateral Flow Assays
The Growing Demand for Rapid, On-Site Diagnostics
The global lateral flow assay (LFA) market is projected to reach $16.8 billion by 2029, driven by the need for point-of-care testing and early disease detection. The demand for fast, accurate, and accessible diagnostic testing has never been higher. From infectious disease detection and food safety to environmental monitoring and veterinary diagnostics, industries are seeking reliable ways to test on-site, in real time.
LFAs have emerged as a go-to solution for point-of-need testing, offering speed, simplicity, and cost-effectiveness. But how can these tests be further improved to meet the increasing demand for higher accuracy, sensitivity, and real-world usability?
This article explores key innovations and best practices to optimize lateral flow assays for maximum performance in point-of-need applications.
What Makes Lateral Flow Assays Ideal for Point-of-Need Testing?
Unlike traditional lab-based diagnostics that require specialized equipment and personnel, LFAs provide:
- Rapid Results: Most lateral flow tests deliver results in under 15 minutes, enabling fast decision-making.
- User-Friendly Design: LFAs are designed for non-experts, making them suitable for field use and at-home testing.
- Portability & Shelf-Stability: These tests are compact, lightweight, and often require no refrigeration.
- Cost-Effectiveness: Compared to traditional lab-based assays, LFAs are affordable and scalable.
Pro Tip
To meet growing industry demands, LFA developers must focus on enhancing sensitivity, specificity, usability, scalability, and regulatory compliance.
Key Innovations to Improve Lateral Flow Assays
While LFAs are already transforming diagnostics, technological advancements can further boost their effectiveness for point-of-need testing.
1. Enhancing Sensitivity with Advanced Detection Methods
One of the biggest challenges in LFA development is improving sensitivity, particularly for detecting low-concentration analytes.
- Gold Nanoparticles: Gold nanoparticles remain the preferred choice for LFAs as they are cost-effective, rugged, scalable, and readable by eye or by reader.
- Alternative Labels: While alternative labels like polystyrene/latex beads, magnetic beads, and fluorescent nanoparticles may offer enhanced signal amplification in lab settings, they often require specialized readers, increased cost, and added complexity — making them less ideal for field-ready diagnostics.
- Enzyme-Based Signal Enhancement: Incorporating enzymatic reactions can boost assay sensitivity. Trade-offs include additional reagents, steps, and equipment, making them less than ideal for point-of-care applications.
- Digital Readers: Relatively inexpensive readers and smartphone-based image analysis tools can quantify weak signals, improving diagnostic accuracy.
2. Multiplexing: Detecting Multiple Targets in a Single Test
In many applications, a single test needs to detect multiple analytes simultaneously. Multiplexing LFAs can:
- Increase efficiency by reducing the number of tests needed
- Lower costs associated with consumables and processing
- Improve diagnostic decision-making with comprehensive results
Example: In infectious disease diagnostics, a multiplex LFA can detect COVID-19, Influenza A, and Influenza B from a single sample—eliminating the need for separate tests.
3. Integrating Connectivity & Digital Analysis for Smart Testing
Modern diagnostics demand connectivity and real-time data integration. Innovations in LFA digitalization include:
- Smartphone-Based Readers: Mobile apps can capture, interpret, and share test results, enabling remote monitoring and better data management.
- Cloud-Connected LFAs: Some next-gen assays sync results to cloud databases, improving surveillance and epidemiological tracking.
- AI-Powered Interpretation: Machine learning can analyze LFA results with greater accuracy than visual interpretation alone.
Example: A recent breakthrough in telemedicine-integrated rapid diagnostics allows patients to take LFAs at home and instantly share results with physicians, accelerating clinical decision-making.
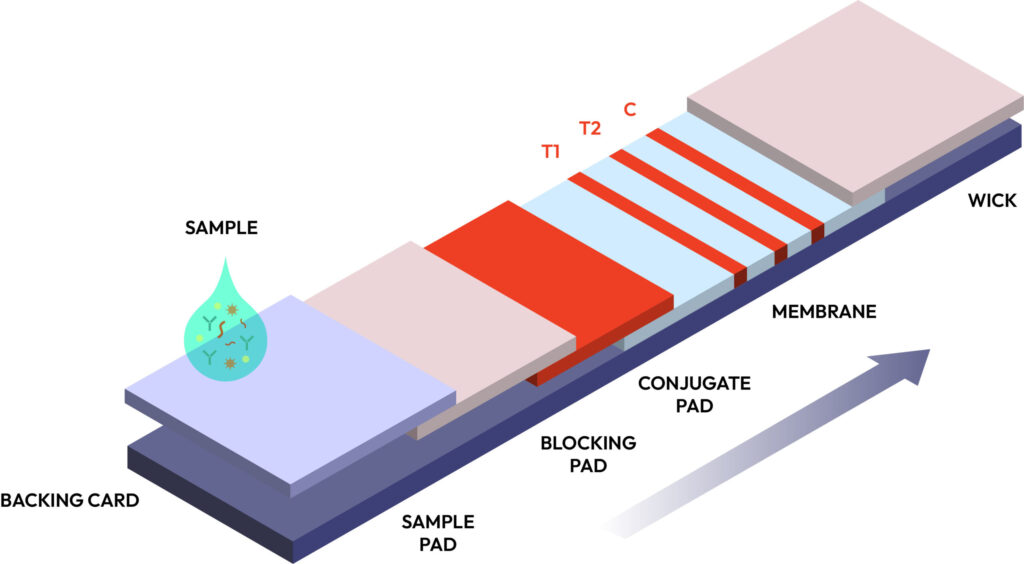
Best Practices for Designing High-Performance LFAs
To maximize impact in point-of-need testing, LFA developers should consider:
- Choosing the Right Sample Matrix: Whether blood, urine, saliva, fecal matter, or environmental samples, optimizing reagents for real-world conditions ensures reliable results.
- Optimizing Membrane & Conjugate Pad Materials: High-quality nitrocellulose membranes improve flow dynamics, reducing background noise and false positives.
- Ensuring Scalability & Manufacturability: Manufacturing consistency is critical for regulatory approval and commercialization success.
Pro Tip
Collaborating with an experienced contract assay development partner ensures a streamlined transition from prototype to scalable manufacturing.
The Future of Lateral Flow in Point-of-Need Testing
The next frontier of point-of-need testing will focus on:
- Ultra-sensitive assays capable of detecting disease at early stages
- At-home testing for chronic disease management beyond infectious diseases
- AI-enhanced rapid diagnostics for automated, real-time decision-making
With ongoing innovations and improvements, lateral flow assays will continue to revolutionize on-site diagnostics across healthcare, food safety, environmental monitoring, and beyond.
Looking to develop or optimize a lateral flow assay for point-of-need testing? BioAssay Works specializes in custom assay development, gold nanoparticle conjugation, and full-scale manufacturing.